EndoDrill® – en banbrytande, marknadsgodkänd biopsimetod för att möjliggöra EUS-CNB
EndoDrill® biopsiinstrument tar vävnadsprover med hög kvalitet och precision med målet att förbättra diagnostiken för flera cancersjukdomar, t ex i magsäck, bukspottkörtel, lever, lunga och urinblåsa. Den innovativa teknologin omdefinierar det växande området för vävnadsprovtagning med endoskopiskt ultraljud för cancerdiagnostik. Den motoriserade roterande EndoDrill®-nålen möjliggör högkvalitativa kärnbiopsier, vilket övervinner begränsningarna med dagens manuella standardnålar.
Under 2023 erhöll EndoDrill® GI, BiBBs inledande produktvariant, marknadsgodkännande från amerikanska FDA som det första eldrivna biopsiinstrumentet för endoskopi i USA. I början av 2024 erhölls CE-certifiering enligt MDR i Europa för hela produktfamiljen EndoDrill®. EndoDrill®-systemet har utformats för att vara enkelt att använda och består av ett sterilt EndoDrill® Biopsy Instrument för engångsbruk med tillhörande EndoDrill® Drive System.
En inledande klinisk pilotstudie, EDMX01, visade på hundraprocentig diagnostisk träffsäkerhet vid analys av prover tagna med EndoDrill® GI
EndoDrill® på 40 sekunder
Investera 40 sekunder och lär dig grunderna om världens första FDA-godkända eldrivna
biopsiinstrument för endoskopiskt ultraljud.

Innovation när den är som bäst
BiBB är ett cancerdiagnostikbolag som utvecklar och tillverkar EndoDrill®, en patenterad produktserie av eldrivna endoskopiska biopsiinstrument.

EndoDrill® / Teknologi
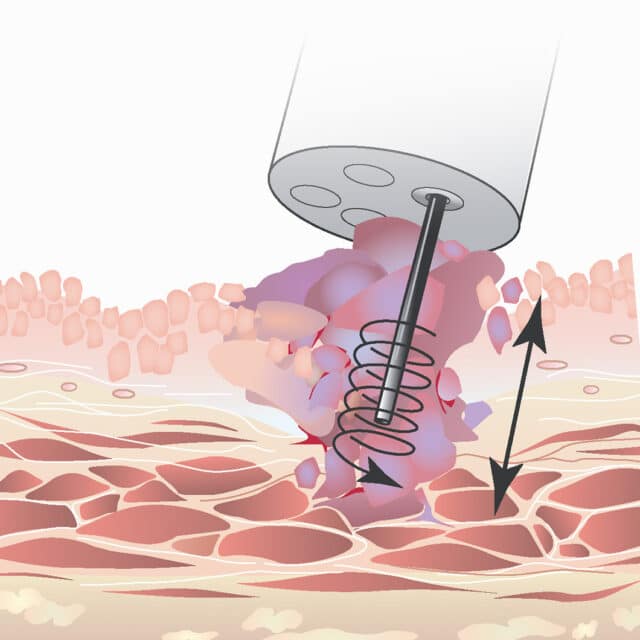
Eldriven rotation är nyckeln till förbättrade biopsier
FDA- och CE- marknadsgodkända EndoDrill® GI nyttjar, till skillnad från konventionella manuella nålinstrument, en eldriven roterande borrcylinder. Designen medger djup provtagning med hög precision. En eller flera högkvalitativa och sammanhängande kärnbiopsier borras ut, vilket krävs för komplett histologisk diagnos, stadieindelning och genetisk analys.
Produktlinjen EndoDrill® består av biopsiinstrument med flexibel borrcylinder (engångsdel) samt drivsystem (flergångsdel) med tillhörande motorenhet, fotpedal och drivkabel. EndoDrill® har utvecklats för att ta vävnadsprover av högsta möjliga kvalitet vid ultraljudsledda endoskopiundersökningar (EUS/EBUS).

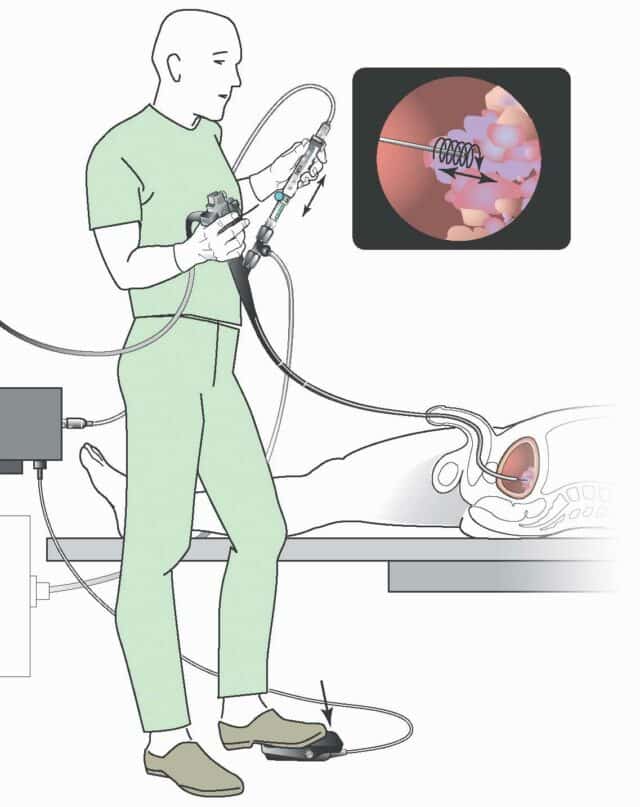
Så här fungerar EndoDrill®-systemet
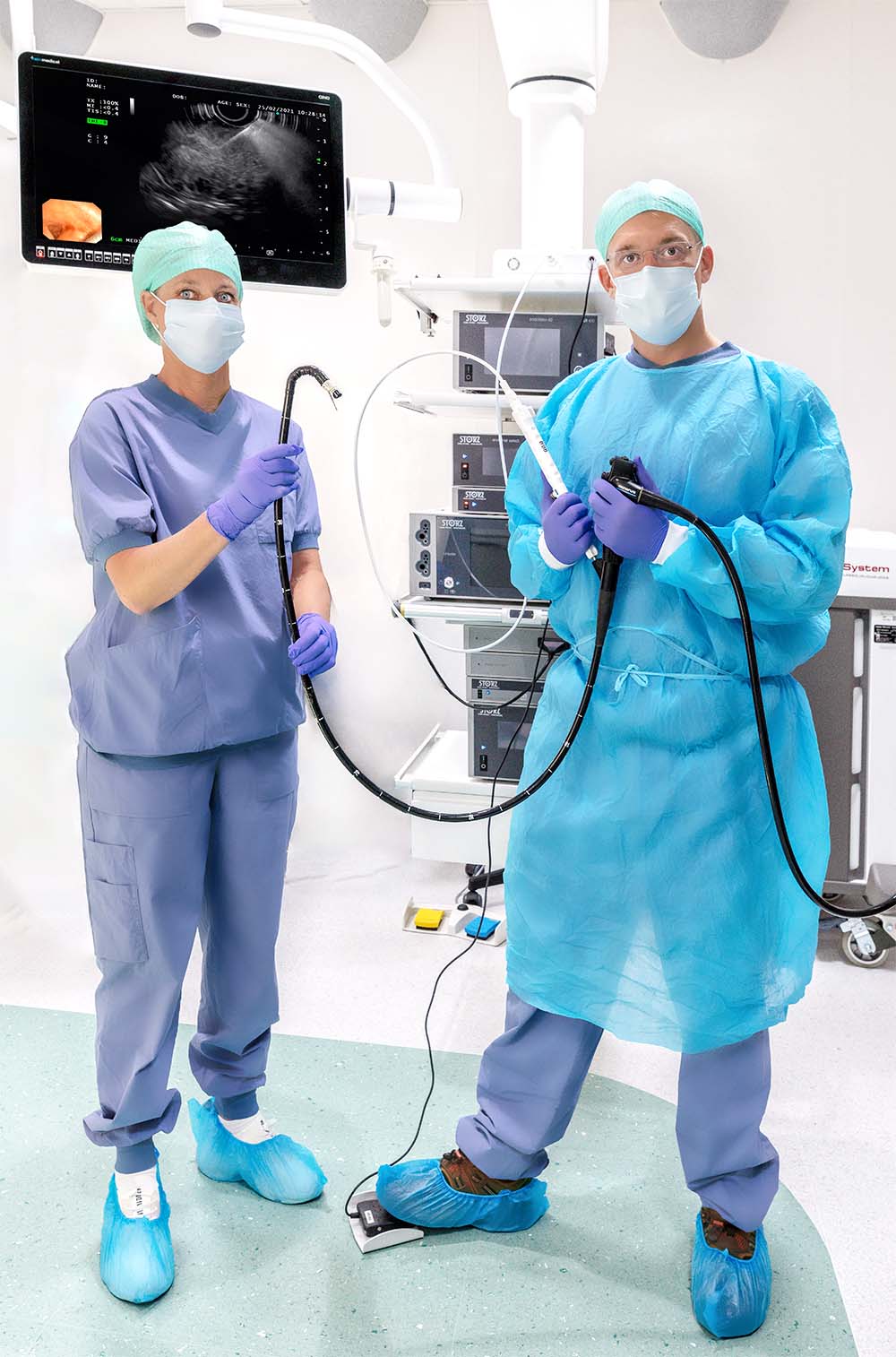
Lär dig mer om EndoDrill®-systemet genom att markera valfri plusikon i bilden.
EndoDrill® Motor Unit
Kompakt motorenhet som kopplas till EndoDrill® Drive Cable och styrs via EndoDrill® Foot Pedal (on/off). Motorn genererar en rotation som överförs till EndoDrill® Drive Cable och vidare till EndoDrill® Biopsy Instruments nålcylinder.
Roterande nålcylinder
EndoDrills eldrivna roterande nålcylinder skär ut solida kärnbiopsier med hög precision för högsta möjliga diagnostiska träffsäkerhet. Den inre flexibla nålcylindern skyddas av ett yttre hölje.
EndoDrill® Drive Cable
EndoDrill® Drive Cable överför rotation från EndoDrill® Motor Unit till EndoDrill® Biopsy Instrument:s inre nålcylinder.
EndoDrill® Biopsy Instrument (engångs)
Intuitivt EUS-provtagningshandtag inklusive låsskruvar med flexibel inre roterande nålcylinder och yttre skyddande hölje.
EndoDrill® Foot Pedal
Med EndoDrill® Foot Pedal styrs motorrotationen (on/off)
Övervinn begränsningarna hos dagens biopsinålar
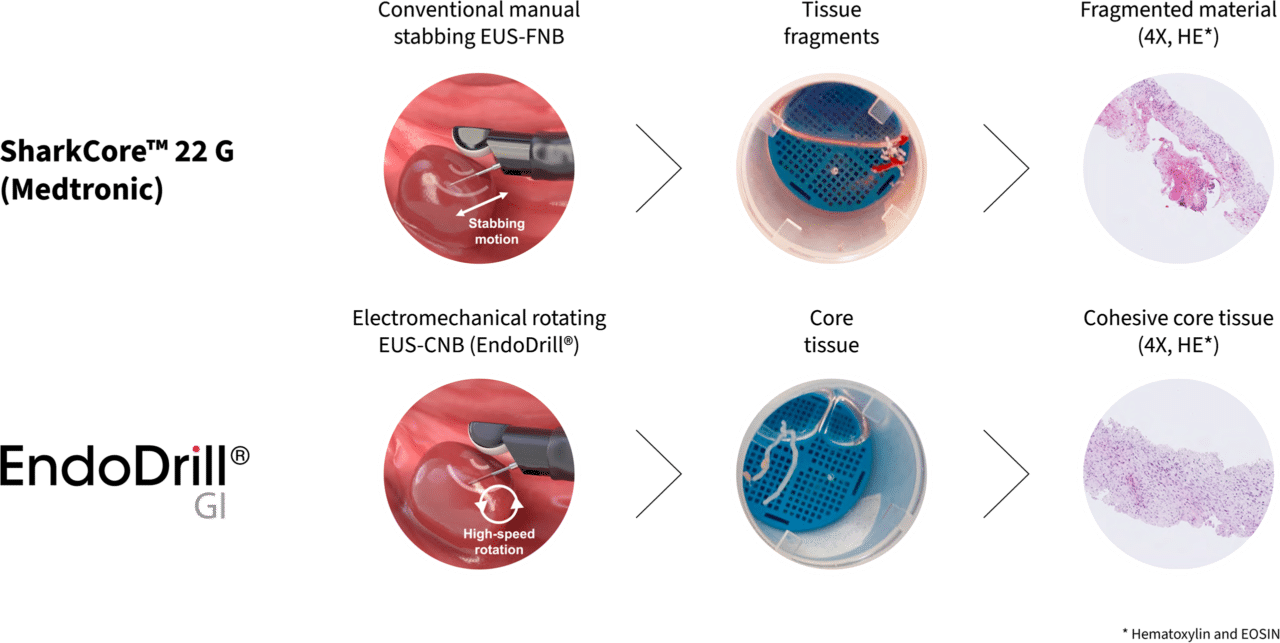
Dagens manuella EUS/EBUS-nålinstrument förs in i tumören med en upprepad huggande rörelse och i nålens spets fastnar lösryckta celler och vävnadsfragment. Metoden kräver erfarna endoskopister för provtagningen och duktiga patologer för utvärderingen. För att öka chanserna att det fragmenterade cellmaterialet leder fram till diagnos är det inte ovanligt att utnyttja olika tilläggsmetoder, t ex nyttja en patolog i salen för realtidsutvärdering (ROSE), centrifugera provet (cellblock), och använda olika tekniker för att aspirera cellprovet. EndoDrill® förenklar hela den diagnostiska processen. I stället för den manuella provtagningen med huggande rörelser tas prover med hjälp av eldriven och användarvänlig precisionsborrning.
Den nya eran med invividanpassad cancerbehandling kräver mer högkvalitativa kärnbiopsier för histologisk diagnos och genetisk analys.

Med en roterande flexibel nålcylinder skär endoskopisten ut fina kärnprover utan blodtillblandning och med bibehållen vävnadsarkitektur under hög precision. Provtagningen med EndoDrill® blir mer reproducerbar och standardiserad, d v s mindre beroende av endoskopistens erfarenhet och skicklighet.
En annan mycket viktig och unik egenskap är den ultraflexibla designen som möjliggör provtagning med kraftigt vinklat endoskop. Det innebär att man kan ta högkvalitativa prover på mycket svårtillgängliga tumörer, vilket många gånger är tekniskt besvärligt med dagens styvare biopsinålar.
Ultraflexibel nål ger enkel åtkomst även till
svåra anatomiska positioner.
EndoDrill® jämfört med manuell provtagning med finnålar
|
EndoDrill® |
Dagens vårdstandard |
|
|---|---|---|
| Vävnadsprovets kvalitet | Solida kärnbiopsier (CNB) med bevarad histologisk vävnadsarkitektur resulterar i hög diagnostisk noggrannhet2,3 4 | Blodtillblandad röra av lösryckta celler och vävnadsfragment (FNA/FNB) av varierande kvalitet |
| Vävnadsprover lämpliga för | För både histologi och genetisk analys1,2,3 | Varierar kraftigt, beroende på t.ex. operatörens erfarenhet, från enbart cytologisk diagnos till en mer fullständig diagnos |
| Tidsåtgång | Potentiellt kortare procedur med eldriven rotation, färre nålpunktioner, färre prover krävs3 | Tidsödande med multipla, manuellt utförda nålpunktioner, många prover krävs |
| Precision | Hög precision med eldriven ”höghastighetsborrning” | Lägre precision med manuella upprepade, kraftfulla nålstick |
| Nålinstrumentets flexibilitet | Ultraflexibelt instrument – fungerar även med kraftigt vinklat endoskop | Styvare instrument krävs för att manuellt penetrera vävnaden – fungerar sämre med böjt endoskop |
| Behov av kompletterande tekniker | Högkvalitativa biopsier erhålls utan hjälptekniker/ROSE1,2,3 | Kräver ofta tillägg av resurskrävande tekniker, t.ex. ROSE och cellblock, för att öka det diagnostiska utbytet |
1. Swahn et al., 2022, EndoDrill® Model X Biopsy Instrument, The Advent of the First EUS Guided 17 Gauge Core Needle Biopsy, Poster session presented at DDW, San Diego.
2. Swahn et al., 2024, The advent of the first electric driven EUS-guided 17 gauge core needle biopsy – A pilot study on subepithelial lesions. Scandinavian Journal of Gastroenterology, 1–7. https://doi.org/10.1080/00365521.2024.2336611
3. Mendoza Ladd A et al. Initial Experience With The Transmural Use Of A New Endoscopic Ultrasound Electric Core Needle Biopsy Device: A Case Series. Endoscopy International Open 2024. doi: 10.1055/a-2427-2311
Med en högkvalitativ kärnbiopsi (CNB, Core Needle Biopsy) redan vid första undersökningstillfället nås målet om en behandlingsgrundande diagnos direkt och resurskrävande metoder och upprepade provtagningstillfällen kan undvikas. Tidig definitiv diagnos med histologisk och genetisk information innebär att personanpassad behandling omgående kan sättas in, vilket kan rädda liv och spara resurser i sjukvården.
Från 2000-talets EUS-provtagning med finnålsaspiration av celler (EUS-FNA) till 2010-talets finnålsbiopsi (EUS-FNB) av vävnadsfragment ges, med introduktionen av eldrivna EndoDrill®, lösningen till 2020-talets provtagning av högkvalitativa kärnbiopsier (EUS-CNB). Det blir en efterlängtad förbättring av diagnostiken för många cancerformer med potential att på sikt etablera en ny vårdstandard för endoskopisk provtagning.

EndoDrill® / Produktportfölj
Nästa generations eldrivna biopsiinstrument
BiBB utvecklar och tillverkar för närvarande tre produktvarianter av EndoDrill® som kan användas för endoskopisk vävnadsprovtagning i sex av de tio vanligaste cancerformerna. EndoDrill®-produkterna är kompatibla med endoskop från de stora tillverkarna och består av sterila EndoDrill® Biopsy Instrument kopplade till EndoDrill® Drive System.
De olika biopsiinstrumenten i produktportföljen är anpassade till varje applikations specifika krav och miljö. EndoDrill® GI (mag-tarmkanalen) och EndoDrill EBUS (luftvägar/lunga) används med ultraljudsendoskop, medan EndoDrill® URO (urinvägar) används med ett konventionellt cystoskop. Det motoriserade EndoDrill® Drive System används för alla biopsiinstrument.
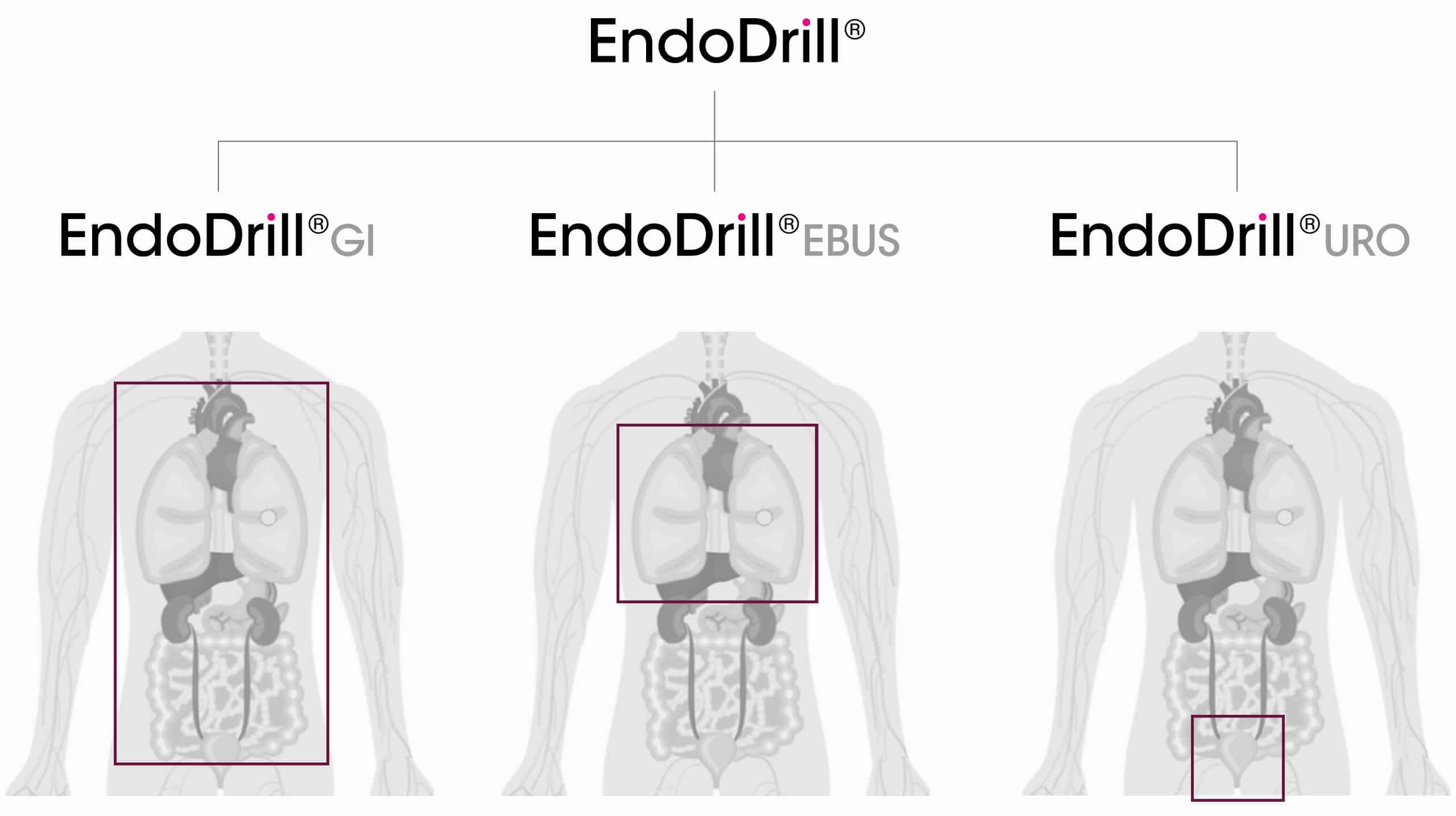
Lär dig mer om EndoDrill®s olika produktvarianter genom att markera valfri plus-ikon i bilden.
EndoDrill® GI
används för ultraljudsstyrd (EUS) provtagning i mag-tarmkanalen och dess närliggande organ, t ex i magsäck, bukspottkörtel, lever och lymfkörtlar.
EndoDrill® EBUS
används i luftvägarna vid provtagning med endobronkiellt ultraljud (EBUS) för diagnos och stadieindelning av lungcancer.
EndoDrill® URO
används med standardcystoskop för tidigarelagd provtagning av muskelinvasiv blåscancer.
EndoDrill® GI
EndoDrill® GI används för provtagning med endoskopiskt ultraljud (EUS) för alla indikationer i mag-tarmkanalen, t ex pankreas, magsäck, matstrupe, lymfkörtlar och lever.
- Utvärderas för närvarande på sjukhus i Europa och USA.
- Retrospektiv multicenterstudie – EndoDrill® GI – effektiv och säker (2025).
- Första försäljningsordern mottagen från ett sjukhus i USA (2025).
- Amerikansk klinisk fallserie publicerad expertgranskad vetenskaplig tidskrift – 100 procents diagnostik noggrannhet (2024).
- Svensk klinisk pilotstudie publicerad i expertgranskad vetenskaplig tidskrift – 100 procents diagnostik noggrannhet (2024).
- Framgångsrik fallserie i USA publicerad i expertgranskad vetenskaplig tidskrift (2024).
- CE-märkt enligt MDR i februari 2024.
- FDA-godkänd för amerikanska marknaden i mars 2023.
EndoDrill® GI är världens första marknadsgodkända eldrivna EUS-CNB-instrument.
EndoDrill® GI – provtagning och resultat från patientfall (#2, EDMX01)
Bildserien visar på EndoDrills visade förmåga att ta behandlingsgrundande vävnadsprover av högsta möjliga kvalitet.
Ladda ner produktblad för EndoDrill® GI
Bruksanvisning, inklusive säkerhets- och prestandainformation som är relevant för användaren eller någon annan person, medföljer produkten eller tillhandahålls på begäran. Vänligen kontakta BiBBInstruments på info@bibbinstruments.com.
EndoDrill® EBUS
EndoDrill® EBUS används i luftvägarna vid provtagning med endobronkiellt ultraljud (EBUS) för diagnos och stadieindelning av lungcancer. I takt med att allt fler målstyrda behandlingar finns tillgängliga så ökar kraven på de diagnostiska vävnadsprovens kvalitet och dagens finnålsinstrument lever inte helt upp till förväntningarna. EndoDrill® EBUS har designats för att svara upp mot de nya kraven på histopatologiska och genetiska analyser. Med rätt diagnos kan man sätta in rätt behandling även vid svåra fall.
- Planerad lansering år 2026
- CE-märkt enligt MDR i februari 2024.
Revolutionerande biopsiinstrument för lungcancer som möjliggör histopatologiska och genetiska analyser
EndoDrill® URO
EndoDrill® URO används med standardcystoskop för provtagning av muskelinvasiv blåscancer (MIBC). Syftet med EndoDrill® URO är att för första gången ta vävnadsprover av djupväxande blåstumörer redan vid den inledande cystoskopin. Med en tidigarelagd diagnos skulle dagens invasiva operativa ingrepp (TURB) kunna undvikas och behandlingen av patienter med MIBC skulle kunna inledas tidigare. En inledande pilotstudie visar att EndoDrill® URO på ett säkert sätt kan ta behandlingsavgörande prover tidigare i vårdkedjan vid misstanke om MIBC.
- CE-märkt enligt MDR i februari 2024,
- Pilotstudie slutförd 2022 och positiva data publicerade i juni 2023.
Möjligt paradigmskifte för diagnostik av muskelinvasiv urinblåsecancer (MIBC)
EndoDrill® / Kliniska studier
Positiva resultat för EndoDrill®
BiBB har genomfört prekliniska jämförelser, en klinisk pilotstudie, en klinisk fallserie samt en retrospektiv multicenterstudie med EndoDrill® GI. Överlag tar EndoDrill® GI sanna kärnbiopsier, oftast redan vid första nålpunktionen. I de fall där EndoDrill® GI jämförts med marknadsledande konkurrenter (EUS-FNB), visar resultaten att EndoDrill® tar vävnadsprover av högre diagnostisk kvalitet. Fortsatta kliniska studier och akademiska samarbeten planeras för att ytterligare stärka den kliniska evidensbasen.
Studie EDMX01 med EndoDrill® GI
Hösten 2020 inleddes den första kliniska studien, EDMX01, på tre svenska universitetssjukhus för provtagning av svårdiagnostiserad cancer i övre mag-tarmkanalen (SEL-tumörer).
Analysen visade entydigt att EndoDrill® på ett säkert sätt tog högkvalitativa vävnadsprover, s k kärnbiopsier, även jämfört med ledande finnålsinstrument (EUS-FNB). Studien presenterades i maj 2022 på DDW-kongressen i San Diego, USA, och publicerades i den expertgranskade tidskriften Scandinavian Journal of Gastroenterology i mars 2024.
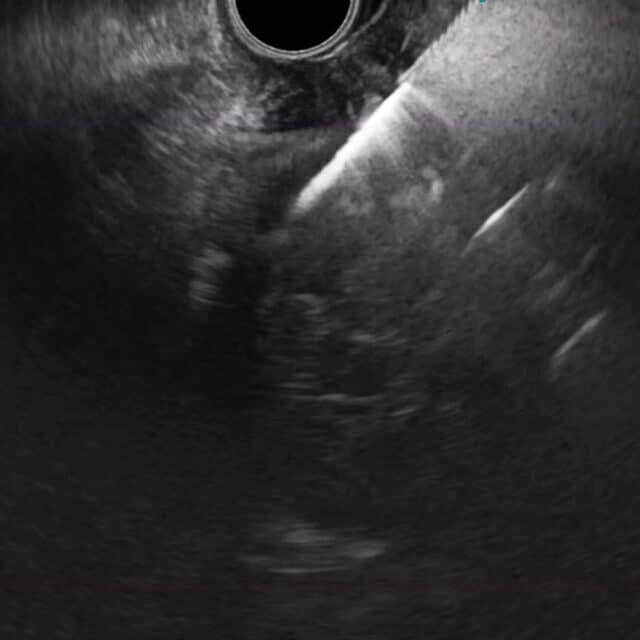
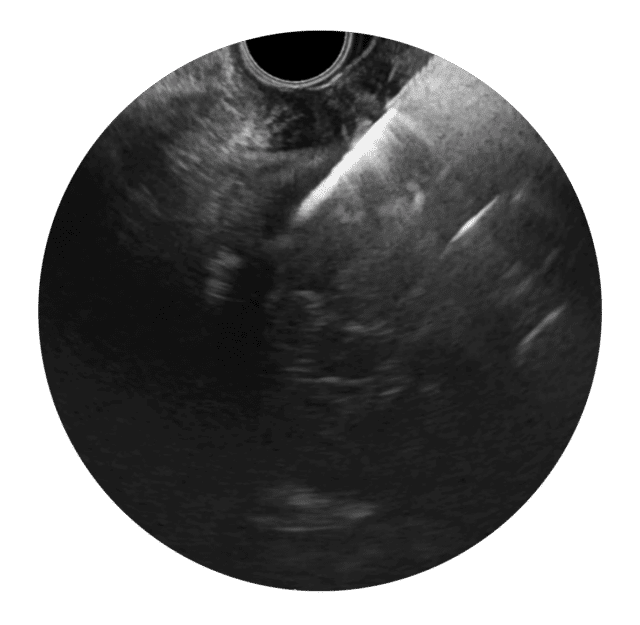
Ultraljudsbilden visar tydligt när EndoDrill® GI borras in i en submukös tumör.
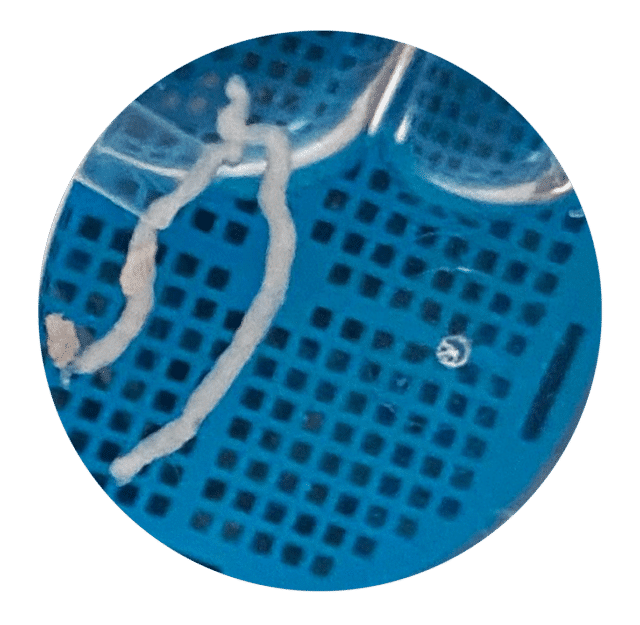
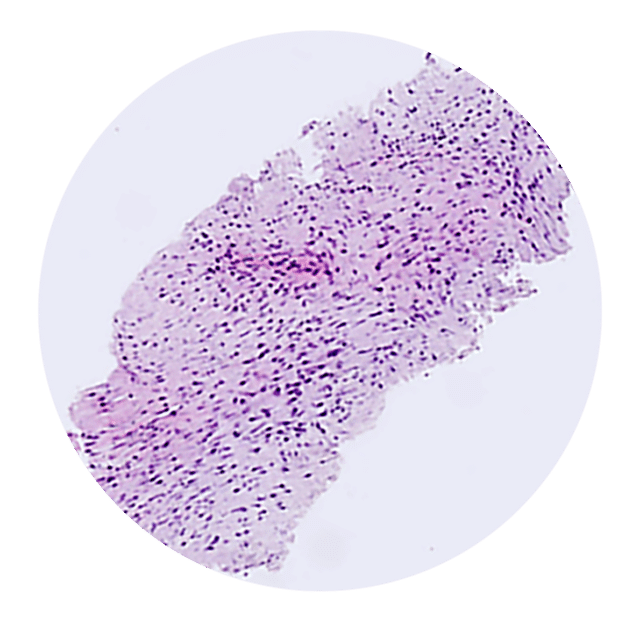
Bildserien nedan från fall 2 i studie EDMX01 visar skillnaden i vävnadsprovets kvalitet när biopsier tas på samma tumör med EndoDrill® GI respektive ledande EUS-FNB-instrument. Stora och sammanhängande vävnadsprover innehåller mer information och förbättrar diagnostisk noggrannhet .
* Swahn et al, 2022, EndoDrill® Model X Biopsy Instrument, The Advent of the First EUS Guided 17 Gauge Core Needle Biopsy, Poster session presented at DDW, San Diego.
Länk till den vetenskapliga artikeln i Scandinavian Journal of Gastroenterology
Fallstudier våren 2024 med EndoDrill® GI i Europa och USA
Efter erhållande av FDA 510(k)-godkännande och CE-märkning (MDR) för EndoDrill® GI påbörjades fallstudier vid flera sjukhus i Skandinavien och USA under våren 2024. EndoDrill® GI används för provtagning med endoskopiskt ultraljud i mag-tarmkanalens organ. Utvärderingarna har skett på många olika tumörformer och har börjat väldigt positivt. Nedan syns ett urval av högkvalitativa kärnbiopsier som BiBBs team dokumenterat på plats i undersökningsrum.
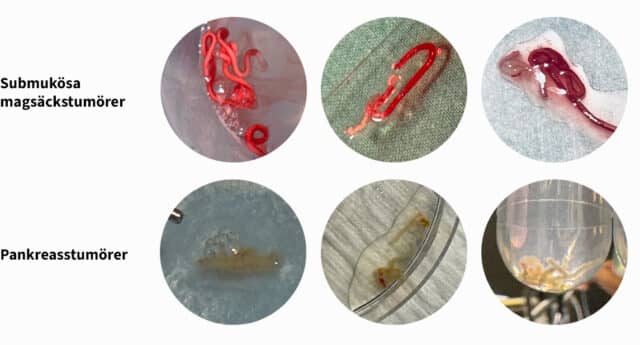
Klinisk fallserie i USA visar 100% diagnostisk noggrannhet med EndoDrill GI
I januari 2024 inledde dr Antonio Mendoza Ladd, medicinsk chef för endoskopi vid UC Davis Health i Sacramento, Kalifornien, en klinisk utvärdering av EndoDrill® GI som fortfarande pågår. Resultaten av de första åtta patientfallen publicerades i slutet av september 2024 i en artikel; ”Initial Experience With The Transmural Use Of A New Endoscopic Ultrasound Electric Core Needle Biopsy Device: A Case Series“ i Endoscopy International Open1.
Dessa patientfall representerar de första transmurala (genom väggen på mag-tarmkanalen) provtagningarna med ett EUS-CNB-instrument (EndoDrill® GI) med prov i tumörer i bukspottkörteln (n=5), retroperitoneum (n=2) respektive mediastinum (n=1). I samtliga åtta patientfall kunde diagnos fastställas med hjälp av vävnadsbiopsier tagna med EndoDrill® GI (100 % diagnostisk noggrannhet) efter ett enda nålstick. I fyra av fallen hade patienterna först provtagits med manuella EUS-FNA/FNB-nålinstrument (dagens ”gold standard”) vilka gett otillräckliga vävnadsprover för att säkerställa diagnos. I samtliga av dessa fallen resulterade vävnadsprover tagna med EndoDrill® GI i fullständig diagnos. Proverna med EndoDrill® GI visade på mindre blodkontaminering, mindre artefakter och mer intakta vävnadskärnor än vad som vanligtvis ses vid provtagning med standard EUS-FNA/FNB-instrument. Den enda noterade biverkningen var ett fall av mild blödning, som kontrollerades framgångsrikt.
Författarnas intryck efter de första fallen med EndoDrill® GI är att provtagningsmetoden är effektiv och säker samt enkel att installera och använda. De avslutar med att rekommendera en randomiserad klinisk studie som jämför EndoDrill® GI med standard EUS-FNA/FNB-nålinstrument för att ytterligare bedöma produktens effekt och säkerhet.
1 Mendoza Ladd A, Alsamman A, Meiklejohn K et al. Initial Experience With The Transmural Use Of A New Endoscopic Ultrasound Electric Core Needle Biopsy Device: A Case Series. Endoscopy International Open 2024. doi: 10.1055/a-2427-2311
Fallserien presenterades även vid en postersession på ACG 2024 i Philadelphia den 29 oktober 2024.
Ladda ner postern här
Retrospektiv analys sammanfattar att EndoDrill GI är säker och effektiv
En retrospektiv analys genomförd i USA och Europa presenteras vid ESGE Days i Barcelona den 3 april 2025. Studien inkluderar data från användning av EndoDrill® GI i klinisk praxis för provtagning av kärnvävnad hos 28 patienter vid fem universitetssjukhus i USA och Europa. Studien inkluderade 28 patienter som genomgick biopsiprovtagning med EndoDrill® GI vid fem universitetssjukhus i Sacramento, Bergen, Stockholm, Linköping och Zagreb. De vanligaste tumörlokalisationerna var bukspottkörteln, retroperitoneum och magsäcken. Hos 22 patienter var den första och enda nålpunktionen med EndoDrill® GI tillräckligt för att ge diagnostiskt vävnadsmaterial.
Författarna drar slutsatsen (översatt till svenska): ”Den övergripande initiala erfarenheten med detta nya EUS-CB-instrument [EndoDrill® GI] var positiv. Våra resultat tyder på att enheten är effektiv och säker. Dess effektivitet när det gäller att erhålla tillräckligt med vävnad med endast en nålpunktion kan potentiellt förkorta den totala procedurtiden. Prospektiva studier som jämför den [EndoDrill® GI] med FNA/FNB-nålar kommer att krävas för att ytterligare bedöma prestanda och säkerhet.
Vill du också testa EndoDrill® GI?
Är du EUS-endoskopist och vill kliniskt utvärdera EndoDrill® GI? Skicka ett mejl till info@bibbinstruments.com så hör vår kliniska supportavdelning av sig. I dagsläget gäller erbjudandet i Europa och USA där produkten är regulatoriskt godkänd (CE-MDR respektive FDA 510(k)).
”This device will be a game changer in my opinion.”
Dr Antonio Mendoza Ladd MD, AGAF, FACG, FASGE Associate Professor of Medicine UC Davis Medical Director of Endoscopy UC Davis Health
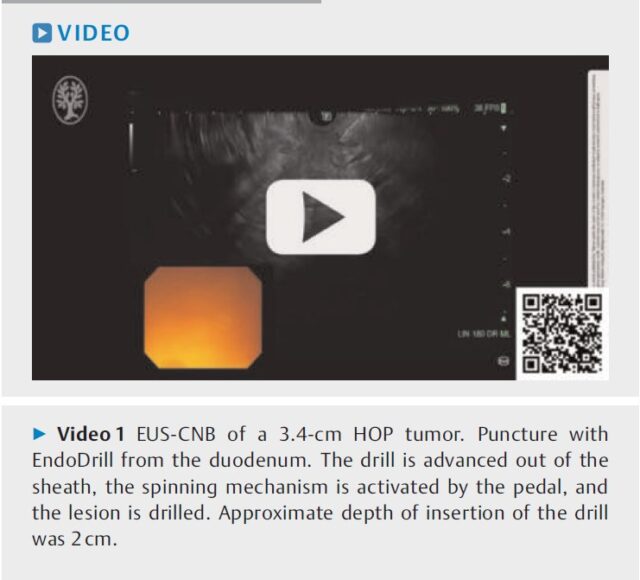
Studie EDUX02 med EndoDrill® URO
BiBB har ännu ett pågående studieprogram för urinvägarna, vilket avser vävnadsprovtagning med EndoDrill® URO i standardendoskop vid muskelinvasiv urinblåsecancer (MIBC), d v s tumörer som vuxit genom urinblåsans slemhinna och muskel. En inledande klinisk studie om tio patienter slutfördes 2022 och resultaten publicerades i tidskriften European Urology Open Science i juni 2023. Det framgår där att EndoDrill® URO på ett säkert sätt kan ta behandlingsavgörande prover tidigare i vårdkedjan vid misstanke om djupväxande tumörer i urinblåsan. Forskargruppen drar slutsatsen att det är motiverat att följa upp pilotstudien med en randomiserad effektstudie, vilken är planerad och regulatoriskt godkänd. Projektet inkluderar även en hälsoekonomisk analys.
Urologernas långsiktiga syfte med det kliniska programmet är att påvisa att tidig provtagning med EndoDrill® URO kan ersätta dagens standardiserade provtagning vid en s k TURB-operation. Hypotesen är att ledtiden från provtagning till behandlingsstart kan reduceras signifikant, vilket kan förbättra överlevnaden vid denna allvarliga tumörform.
”Histologisk verifiering och molekylär klassificering av MIBC är möjliga för prover som samlats in…”
(Eriksson P, et al., Urodrill – a novel MRI-guided endoscopic biopsy technique to sample and molecularly classify muscle-invasive bladder cancer without fractionating the specimen during transurethral resection, European Urology Open Science, Volume 53, July 2023, Pages 78-82)
Studie EDUX02 visar att EndoDrill® URO är det första endoskopiska biopsiinstrumentet som tar behandlingsavgörande prover på ett säkert sätt vid misstanke om urinblåsecancer.
Patent
BiBBs IP-portfölj är en av Bolagets mest kritiska tillgångar. Samtliga av BiBBs produkter har antingen beviljade patent alternativt patent under behandling (patent pending). Det eldrivna EndoDrill®-systemet skyddas av godkända patent i Europa, Japan, Indien och Kina samt tre pågående internationella patentansökningar som har inträtt i nationell fas. Utöver patent så är varumärket EndoDrill® registrerat på de största marknaderna.
| Patentfamilj | Ansökan under behandling | Godkänt patent | Giltighet |
|---|---|---|---|
|
EndoDrill |
Patentfamilj 1 |
||
|
|
|
Europa | 2038 |
|
|
|
Europa II, Japan, Indien, Kina |
2039 |
|
|
|
Europa III | 2039 |
|
|
Patentfamilj 2
|
Japan |
2040 |
|
Patentfamilj 3
|
Kina (NoA) |
2041 |
|
|
EndoDrill (manuell, Gen 1) |
USA, Kanada | 2033 | |
|
Handtaget (manuell, Gen 1) |
Europa, Kina | 2035 | |
|
EndoDrill Core Needle (manuell, Gen 1) |
Sverige | 2038 | |
| EndoDrill® Trademark | USA | Europa, Australien, Kina, Indien, Japan, Kanada |