About BiBB / BiBB in brief
Innovation at its finest
BiBB is a cancer diagnostics company that develops and manufactures EndoDrill®, a patented product line of electric-driven endoscopic biopsy instruments. EndoDrill® works with high precision to take high-quality tissue samples with the aim of improving diagnostics for several types of cancer, such as stomach, pancreatic, liver, lung and bladder cancer. The product portfolio targets the global market for ultrasound-guided (EUS/EBUS) biopsy instruments, which represents the most advanced and fastest-growing segment of endoscopy.
In 2023, EndoDrill® GI, BiBB’s lead product, received market clearance from the US FDA. At the beginning of 2024, BiBB received CE certification according to MDR in Europe for the entire EndoDrill® product family. EndoDrill® is thus the first and only electric-driven biopsy instrument to be market-cleared in both the US and Europe. The EndoDrill® system includes sterile disposable biopsy instruments with an associated drive system (reusable).
BiBB was founded in 2013 by Dr Charles Walther, cancer researcher at Lund University and senior physician in clinical pathology at Skåne University Hospital in Lund. BiBBInstruments is based at Medicon Village in Lund, and the BiBBInstruments share (Ticker: BIBB) is listed on the Spotlight Stock Market.
A Swedish listed medtech company that has developed the world’s first market cleared electric-driven biopsy system for endoscopy.
About BiBB / Business strategy
Business strategy
BiBB’s business model is based on the development and sale of innovative biopsy instruments in the premium segment under the EndoDrill® brand. The company creates value by building a patented product portfolio with three product variants for improved tissue sampling, used for subsequent diagnosis of several common cancer types. The products are intended for public and private hospitals offering advanced endoscopic and endobronchial ultrasound procedures (EUS/EBUS). Revenue is generated from the sale of capital equipment, the EndoDrill® Drive System, but primarily from recurring sales of the accompanying sterile biopsy instrument, the EndoDrill® Biopsy Instrument.
EndoDrill® is the world’s first powered biopsy instrument cleared for tissue sampling with EUS/EBUS. EndoDrill® GI received FDA 510(k) clearance in the U.S. in 2023, and all three product variants – EndoDrill® GI, EndoDrill® EBUS, and EndoDrill® URO – are CE-marked under the MDR in Europe (2024).
A series of successful clinical studies on EndoDrill® GI has been published: a Swedish pilot study (n=7), an American clinical case series (n=8), and a retrospective multicenter study conducted in the U.S. and Europe (n=28). EndoDrill® EBUS for lung cancer is in a late development phase. EndoDrill® URO has been successfully evaluated in an initial Swedish pilot study (n=10), with published data showing proof of concept for the indication of muscle-invasive bladder cancer (MIBC). Together with clinical partners, the company continues to generate scientific data for the most important indications. The extensive patent portfolio, consisting of three patent families, is continuously being strengthened.
BiBB began sales of EndoDrill® GI in January 2025 when a U.S. university hospital, following clinical evaluation, placed an order for the instrument. The goal is to sign agreements with one or more global distribution partners during 2025 to enable rapid international sales growth. The launch of EndoDrill® EBUS is planned for 2026.
A patented, regulatory-approved product portfolio of unique biopsy instruments in the fast-growing EUS/EBUS market positions BiBB as an attractive partner.
Mission
We make innovative endoscopic biopsy instruments that advance cancer treatment.
Our vision is to improve outcomes for cancer patients.
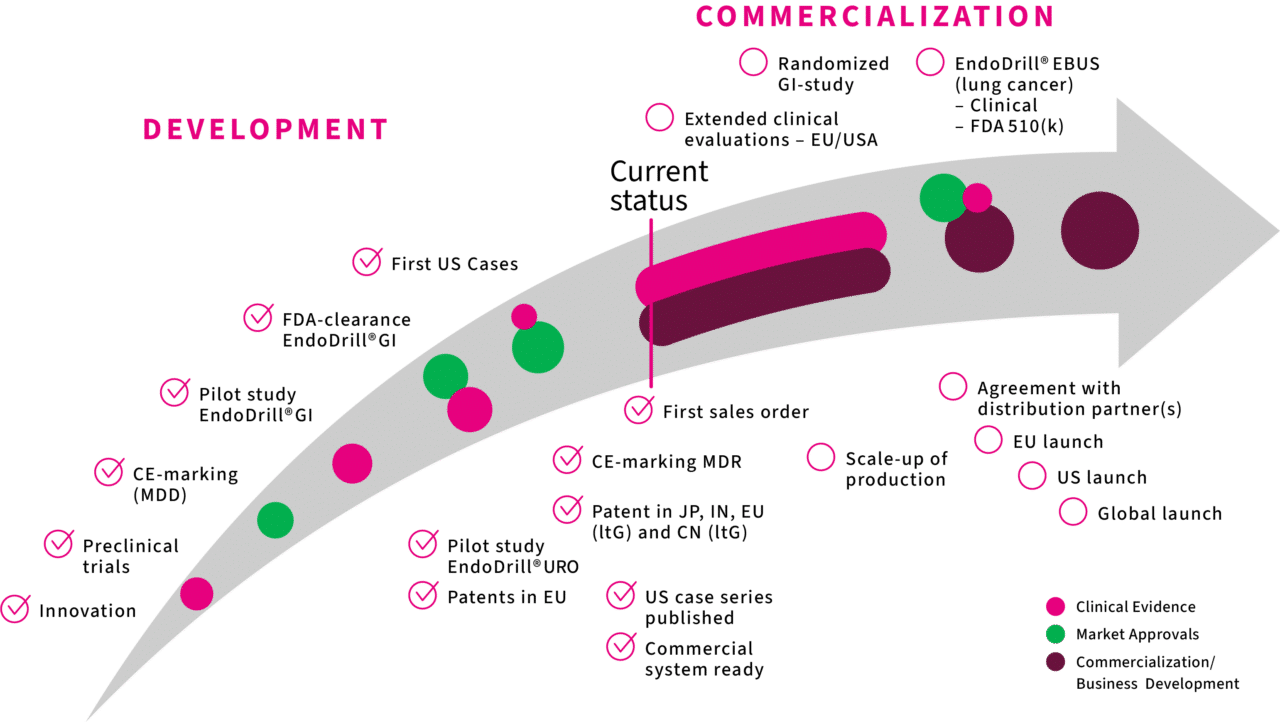
Our journey to commercialization
About BiBB / Market
BiBB’s market – EUS/EBUS biopsy instruments
BiBB’s focus area is ultrasound endoscopy, which is the most advanced form of endoscopy and the fastest-growing segment. For examination of the gastrointestinal tract, it is called endoscopic ultrasound (EUS), while for the airways/lungs, it is called endobronchial ultrasound (EBUS). Ultrasound endoscopy combines visual endoscopy with ultrasound, making it possible to see potentially malignant lesions deeper into the organs and surrounding tissues. Endoscopic ultrasound is used by gastro specialists, while ultrasound bronchoscopy is performed by pulmonary medicine specialists.
The market is dominated by some of the world’s largest medtech companies marketing manual biopsy instruments. More than 1 million biopsy procedures are performed each year with EUS fine needle instruments1; thus it already constitutes a global multi-billion SEK market.
1 Endoscopic Ultrasound Needles Market, Transparency Market Research, 2018.
Endoscopic ultrasound is the fastest growing segment in endoscopy. In the United States, the number of procedures has increased by an average of 21% per year from 2000 to 2019.
EUS-FNA/FNB – tissue sampling in the GI tract
Endoscopic ultrasound is used by EUS endoscopists to diagnose suspected tumour lesions. Due to its minimally invasive nature and ease of use, EUS has become the fastest growing endoscopic segment for an increasing number of indications. In addition to diagnostic examination, EUS has become important for staging and as a therapeutic method, such as the drainage of pancreatic cysts.
Ultrasound-guided samples are taken endoscopically with manually-handled needles, either fine needle aspiration (EUS-FNA, cytological sample) or biopsy (EUS-FNB, histological sample). The samples are sent to a pathological laboratory for further diagnostics. The method has evolved rapidly over the last two decades and is now used for suspected cancers of the gastrointestinal tract, such as the stomach, pancreas and liver. At pace with the growth of modern personalised treatment of various forms of tumours, increasing demands are being placed for accurate diagnosis prior to the start of treatment. High-quality samples are needed for diagnosis and staging, and to determine personalised treatment (size-reducing therapy, surgery, chemotherapy, radiation, etc.).
Even though ultrasound-guided needle instruments have been refined in recent decades, they usually only capture tissue fragments or tinged material with detached cells. The lack of high-quality solid core biopsies is the missing link in the cancer diagnostics chain.
Read about BiBB’s new biopsy instrument, EndoDrill® GI EndoDrill® GI
EBUS-TBNA – airway/lung and mediastinal tissue sampling
Ultrasound in combination with visual bronchoscopy is used in suspected lung cancer and is then called EBUS, endobronchial ultrasound. Today, EBUS is the diagnostic foundation when investigating suspected lung cancer and is used by pulmonologists for both diagnosis and staging. Once the tumour suspected lesion is localised, ultrasound-guided endobronchial fine needles (EBUS-TBNA) can be used via the airways for tissue sampling.
The principle is the same as in EUS biopsy, but the needle instruments are adapted to the EBUS endoscopes. The aim of the needle biopsy is to diagnose and determine the type of cancer. EBUS-TBNA is now the preferred method for treatment-determining staging of lymph nodes in the mediastinum, the hard-to-reach space between the lungs. Staging provides guidance for treatment and assessment of prognosis. It is sometimes necessary to map out a larger area for complete staging. In such case, endobronchial ultrasound of the airways (EBUS-TBNA) and endoscopic ultrasound of the oesophagus (EUS-FNA/FNB) are combined.
Therapeutic EUS – a new emerging field
EUS has recently evolved from a diagnostic tool to a versatile therapeutic method for the biliary tract, pancreas and liver in particular. This is a logical evolution. If you can reach the tumour and take tissue samples assisted by EUS, you can also perform local treatment.
The trend now is for more and more interventions to be minimally invasive with therapeutic EUS. EUS therapeutic instruments have been developed for interventions such as resection, drainage, stent placement, and drug injection.
Please note that the current EndoDrill® device is not cleared for therapeutic use.
About BiBB / History
The missing link in the
cancer diagnostics chain
BiBB was founded in 2013 by Dr Charles Walther, cancer researcher at Lund University and senior physician in clinical pathology at Skåne University Hospital in Lund. Charles saw insufficient biopsy samples under the microscope on a daily basis. At the same time, new targeted cancer treatments were emerging that required more and more information from the tissue samples collected. In conventional manual ultrasound-guided needle biopsy, the endoscopist punches out a mixture of blood, detached cells, and tissue fragments with a stabbing needle instrument. Charles could clearly see that a crucial link in endoscopic diagnostics was missing in the form of information-rich solid tissue samples (“core biopsies”).
The idea for the electric EndoDrill® was born while Charles was out running. Why not use motorised needle rotation (“Drill”) in endoscopic (“Endo”) sampling? Instead of the usual manual sampling, tissue samples could be taken using a precision electric drill. The hypothesis was that a rotating flexible needle cylinder should be able to cut finer, less blood contaminated tissue, while maintaining tissue architecture. These samples could better respond to the increasing demands of emerging personalised cancer treatment.
Ten eventful years have passed from the identification of a clinical need to market approvals and patent for the world’s first electric-driven biopsy instrument for endoscopy. Some of the most important milestones passed are shown in the timeline.

Important milestones
EndoDrill® receives CE marking according to MDR in Europe
The entire product portfolio is market approved in Europe
Feb 2024
Two approved patents in Europe
The patents provide broad protection of the EndoDrill® system until 2038 and 2039, respectively
Sep 2023
EndoDrill® GI FDA cleared in the USA
The first electric-driven endoscopic biopsy instrument with market clearance in the US
Mar 2023
Clinical study EDUX02 MIBC, n=10
The study concerned muscle invasic bladder cancer (MIBC)
2021-2022
Clinical study EDMX01 SEL, n=7
The study concerned deep tumours in the stomach (SEL tumours)
2020-2021
EndoDrill® CE marking (MDD)
Gen 2 – Electric-driven biopsy instrument for endoscopic ultrasound
2020
Sale of EndoDrill® Gen 1
Manual drilling instrument for standard endoscopes
2017-2019
Listing on Spotlight Stock Market
The development company BiBB was listed on the stock exchange in the autumn of 2017
2017
BiBB was founded by Dr Charles Walther
Cancer researcher and consultant in clinical pathology
2013
Board
Dr Charles Walther
(MD, PhD)
Founder of BiBB, Board member, Chief Medical Officer

Show bio
Dr. Charles Walther, born 1974, is a senior consultant in pathology and clinical cytology at Skåne University Hospital in Lund, Sweden. He founded BibbInstruments in 2013 and was CEO until august 2016. Dr. Walther is the inventor of EndoDrill® technology and other tumor biopsy instruments. In clinical practice, his main field is cytology and biopsy sampling. Dr. Walther is a researcher and has conducted his PhD in tumor genetics at the Department of Clinical Genetics at Lund University. As a result of his clinical work and research projects, he has developed new unique methods and instruments for cancer diagnostics. In 2012 Dr. Walther won TV4’s “The Inventors” with the first version of the EndoDrill® instrument.
Charles Walther (via Company) owns 3,530,000 shares in BiBBInstruments AB (publ), corresponding to approximately 8.8% of the votes and capital in the Company. Charles Walther holds 100,000 warrants, series 2023/2028:B.
Sara Lindroth
(M.Sc.BA)
Board Member

Show bio
Sara Lindroth (born 1969) has been an independent board member of BiBB since November 2018. Sara Lindroth holds an M.Sc.BA and has over 20 years of experience in international marketing and sales for both start-ups and large companies focused on medicine and medical devices. She has been responsible for strategic marketing as well as market and product portfolio development of innovative medical devices in the build-up phase at the international level.
Sara Lindroth currently serves as Managing Director at Jolife AB, a wholly owned company of Stryker Inc., and has global market responsibility for Circulatory Solutions. Previous employment includes Jomed, BlueMedical, Millimed and Astra.
Sara Lindroth privately owns 146,976 shares in BiBBInstruments AB (publ), corresponding to approximately 0.4% of the votes and capital in the Company.
Dr Stephan Dymling
(M. Sc., PhD)
Board member, Chief Technology Officer

Show bio
Dr. Stephan Dymling was born in 1955 and has since September 2013 been member of the Board of BiBBInstruments. Stephan Dymling has experience from several senior positions in medical technology companies for cancer treatment and other therapeutic areas, such as Siemens Life Support Systems (today part of Getinge), ProstaLund, SpectraCure, Medical Vision and Clinical Laserthermia System. Dr. Dymling has specialized in the development of medical equipment and also has a background as head of the biomedical department at Skåne University Hospital in Lund.
Stephan Dymling owns 427,730 shares in BiBBInstruments AB (publ), corresponding to approximately 1.1% of the votes and capital in the Company. Stephan Dymling holds 100,000 warrants, series 2023/2028:B.
Erik von Schenck
(M. Sc., MBA)
Chairman of the Board

Show bio
Erik von Schenck, born 1964, has since July 2017 been Chairman of BiBBInstruments AB. Mr von Schenck has over 20 years of experience of startups, medium-sized companies and major international medtech companies. Erik von Schenck is Vice President and General Manager of Circulatory Solution in American Physio Control, now part of Stryker, and has previously held senior positions in Gambro AB and as CEO of Jostra. Other board assignments include Chairman of Avidicare AB and board member of a number of medtech companies, including Xvivo Perfusion.
Erik von Schenck owns via Schenck Consulting AB and family 178 882 shares in BiBBInstruments AB corresponding to approximately 0.7 % of votes and capital in the Company.
Management
Fredrik Lindblad
(MSc. Engineering, BSc. BA)
CEO

Show bio
Fredrik Lindblad, born in 1967, has been CEO of BiBBInstruments since August 2016. Prior to this, he served as a board member from September 2013 to August 2016. Lindblad has a background in marketing and business development with a focus on early-stage life science companies. Fredrik Lindblad served as CEO of BioActive Polymers 2011–2017 and CEO of QuickCool AB 2004–2010. Lindblad served as Head of Marketing for the medtech company Jostra AB (part of the Getinge Group) 2000–2004.
Fredrik Lindblad owns 602,005 shares in BiBBInstruments AB (publ), corresponding to approximately 1.5% of the votes and capital in the Company. Fredrik Lindblad holds 300,000 warrants, series 2023/2028:A.
Josefin Arborelius
(MSc. Engineering)
R&D Director

Show bio
Josefin Arborelius (born in 1986) joined the company in September 2024 and assumed the role of R&D Director in January 2025. She holds a Master of Science in Mechanical Engineering and has extensive experience in the medtech industry. Previously, she worked as a project manager and product owner at Baxter.
osefin Arborelius does not own any shares in the Company.
Dr Paul Rasmusson
(M. Sc., Ph.D. Business Administration)
Supply Chain Director

Show bio
Paul Rasmusson (born in 1976) joined the company as an advisor in 2022 and assumed the role of Supply Chain Director in March 2025. He holds a doctor’s degree in Business administration with a focus on strategic management and supply chain. Paul has close to 20 years of experience in the medical device industry spanning from start-up operations and business development, through acquisitions and integrations, to senior leadership roles in global medical device companies with his latest role in Stryker in European Supply Planning and Process Transformation.
Paul Rasmusson owns 261,256 shares privately and through companies, corresponding to approximately 0.6% of the votes and capital in the Company.
Dr Charles Walther
(MD, PhD)
Founder of BiBB, Board member, Chief Medical Officer

Show bio
Dr. Charles Walther, born 1974, is a senior consultant in pathology and clinical cytology at Skåne University Hospital in Lund, Sweden. He founded BibbInstruments in 2013 and was CEO until august 2016. Dr. Walther is the inventor of EndoDrill® technology and other tumor biopsy instruments. In clinical practice, his main field is cytology and biopsy sampling. Dr. Walther is a researcher and has conducted his PhD in tumor genetics at the Department of Clinical Genetics at Lund University. As a result of his clinical work and research projects, he has developed new unique methods and instruments for cancer diagnostics. In 2012 Dr. Walther won TV4’s “The Inventors” with the first version of the EndoDrill® instrument.
Charles Walther (via Company) owns 3,530,000 shares in BiBBInstruments AB (publ), corresponding to approximately 8.8% of the votes and capital in the Company. Charles Walther holds 100,000 warrants, series 2023/2028:B.
Lotta Ljungberg
(MSc. Engineering)
Director QA/RA

Show bio
Lotta Ljungberg, born in 1966, has been QA&RA Director at BiBBInstruments AB since October 2022. Lotta Ljungberg has extensive experience in quality management and regulatory work for market expansion and product registrations of medical devices. Ljungberg has held senior positions at international medtech companies, such as HemoCue, CellaVision, Elos Medtech and Biora.
Lotta Ljungberg owns 120,000 shares in BiBBInstruments AB (publ), corresponding to approximately 0.3% of the votes and capital in the Company. Lotta Ljungberg holds 100,000 warrants, series 2023/2028:A.
About BiBB / Career
Unfortunately, we do not have any vacancies at the moment.